Abstract
Introduction: The study retrospectively analyzed data from phase III clinical trials (NCT00689936) to establish a predictive model (for ease of expression named Model 1;predictors: ECOG, β2-microglobulin, hemoglobin, lactate dehydrogenase)for the risk of early grade ≥3 infections in patients with multiple myeloma not eligible for transplant in 2018. Similar predictive models appeared in 2022(a simple score to predict early severe infections in patients with NDMM, Model 2(predictors: albumin, male sex, ECOG, non-IgA type MM); a risk assessment model for early grade≥ 3 infections in patients with NDMM, Model 3(predictors: ECOG, serum β2-microglobulin, globulin, hemoglobin)). This study aimed to determine whether three predictive models could be used to stratify early severe infections in real-world patients from China.
Methods: 223 Patients with multiple myeloma in the Third Hospital of Shanxi Medical University from 1 May 2015 to 30 April 2022 were collected and evaluated based on the three different predictive models and the differences in infection rates among patients grouped by different models were analyzed. Cumulative incidence of early ≥ grade 3 infection was estimated using the Kaplan-Meier method and log-rank test to assess the statistical significance of the difference. To compare the predictive performance in the prediction of infection, the ROC curve was used to show the area under the curve (AUC), and the DeLong's test was used to analyze the difference in AUC.
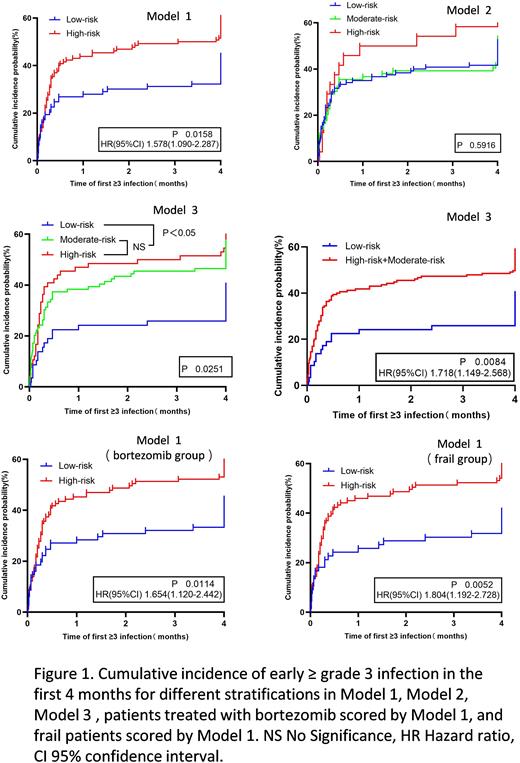
Results: In Model 1,the probability of early ≥ grade 3 infection was significantly higher in the high-risk group than in the low-risk group (60.0% vs 44.1%, P=0.019). In Model 2,the probability of early ≥ grade 3 infection in the low-risk group, medium-risk group, and high-risk group had no statistically significant difference (51.7% vs 53.2% vs 62.5%, P=0.622). In Model 3,the probability of early ≥ grade 3 infection in the low-risk group, medium-risk group, and high-risk group had statistically significant difference (39.7% vs 56.6% vs 60.6%, P=0.044). Cumulative incidence of early ≥ grade 3 infection suggested that patients in the high-risk group had a higher likelihood of occurring early severe infection in the first 4 months in Model 1. In Model 2, no statistically significant differences in the probability of infections were observed among the different groups. There were no statistical differences between moderate-risk and high-risk groups (P=0.555) in Model 3, yet the high-risk (P=0.008)and moderate-risk groups(P=0.023)were substantially different from the low-risk groups(as shown in Figure 1). Comparing the predictive performance of the three models, the AUC of the three models was 0.633, 0.610,0.603(P >0.05). Model 1 was the clinical trial established based on the patients who received thalidomide and lenalidomide-based therapy, yet the patients of our center were mostly taking bortezomib-based therapy and were mostly frail. Consequently, a subgroup analysis was carried out. Subgroup analysis suggested a statistically significant difference in infection of patients treated with bortezomib (P=0.004) and a statistically significant difference in infections in frail patients (P=0.002) in Model 1. In patients treated with bortezomib and frail patients, the high-risk group had a significantly shorter time to early grade ≥3 infections compared with the low-risk group in Model 1(as shown in Figure 1).
Conclusions: A predictive model based on ECOG, β2-microglobulin, hemoglobin, and lactate dehydrogenase (Model 1) offers superior use value and discrimination, including frail patients and those being treated with bortezomib.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal